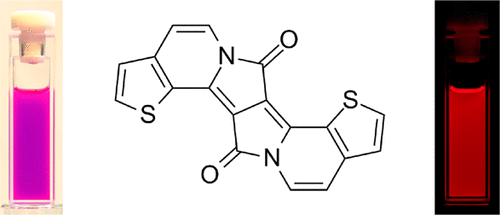
-
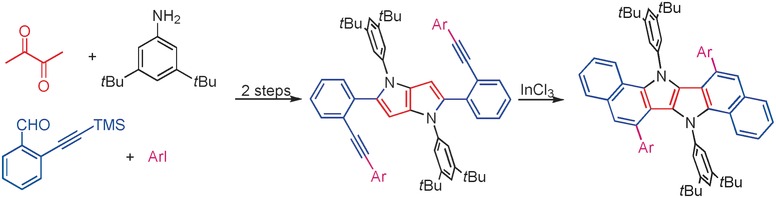
Polysubstitution of dipyrrolonaphthyridinediones as a potent strategy towards strongly emitting fluorophores
Kamil Skonieczny, Łukasz Kielesinski, Marek Grzybowski, Daniel T Gryko
Chem. Commun. 2025, 61, 10178-10181
DOI: 10.1039/D5CC01880C
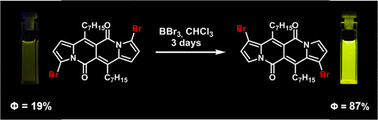
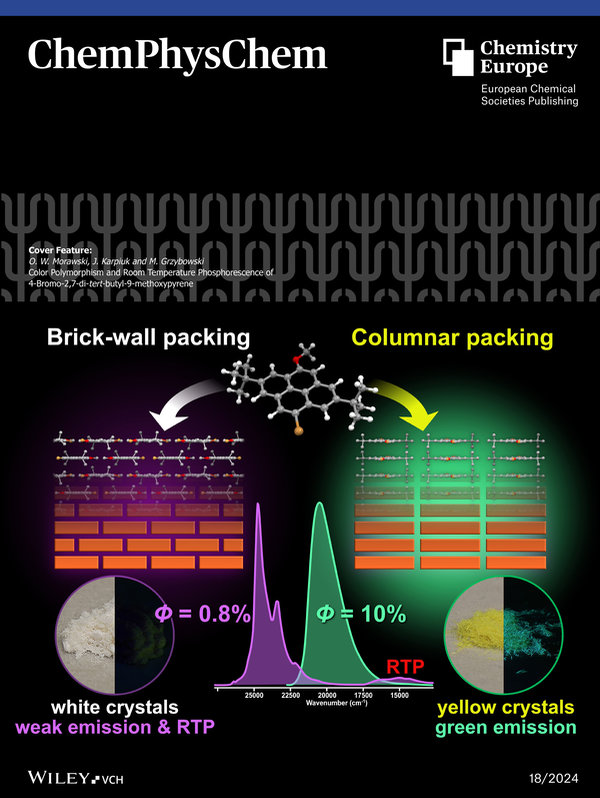
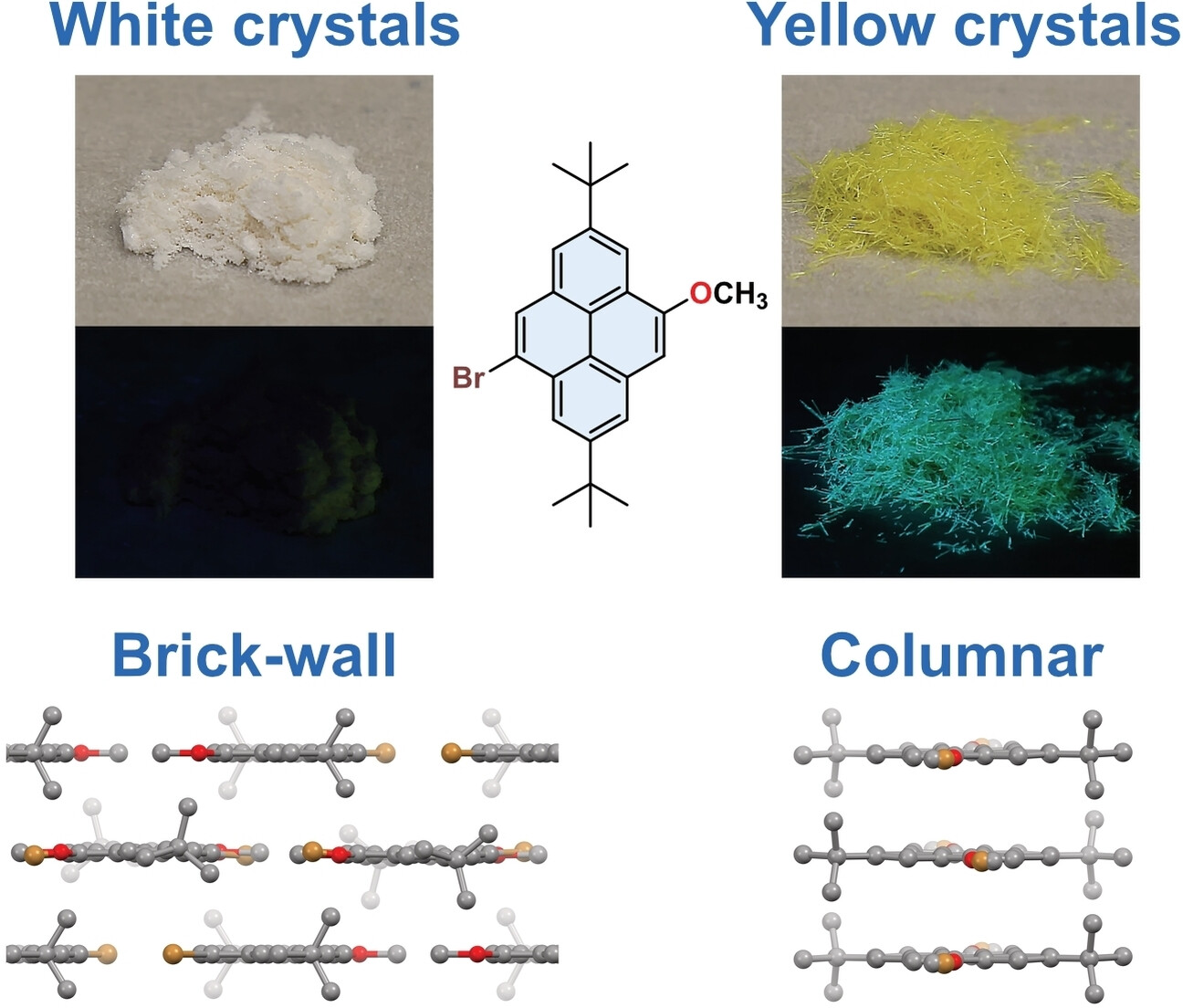
-
Color Polymorphism and Room Temperature Phosphorescence of 4-Bromo-2,7-Di-Tert-Butyl- 9-Methoxypyrene
Olaf W. Morawski, Jerzy Karpiuk, Marek Grzybowski
ChemPhysChem 2023, 25, e202400457
DOI: 10.1002/cphc.202400457
-
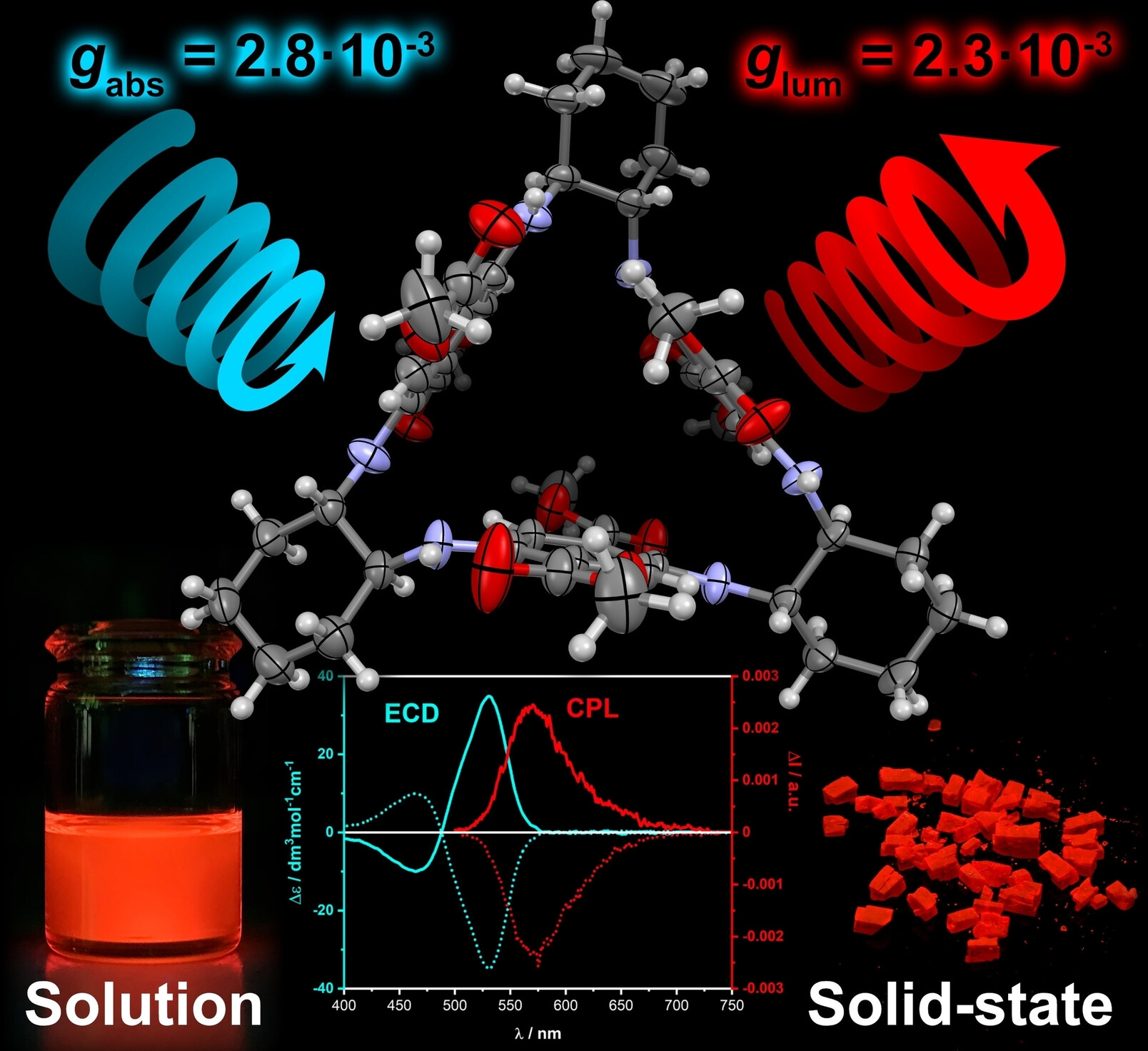
Strong Chiroptical Effects in the Absorption and Emission of Macrocycles Based on the 2,5-Diaminoterephthalate Minimal Fluorophore
Krzysztof Nowak, Olaf Morawski, Francesco Zinna, Gennaro Pescitelli, Lorenzo Di Bari, Marcin Górecki, Marek Grzybowski
Chem. Eur. J. 2023, 29, e202300932
DOI: 10.1002/chem.202300932
-
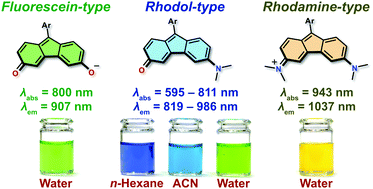
Fluorene analogues of xanthenes – low molecular weight near-infrared dyes
Marek Grzybowski, Olaf Morawski, Krzysztof Nowak, Paula Garbacz
Chem. Commun. 2022, 58, 5455-5458
DOI: 10.1039/D2CC00561A
-
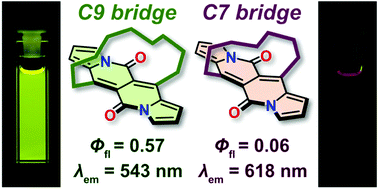
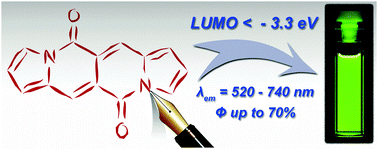
Tuning the aromatic backbone twist in dipyrrolonaphthyridinediones
Bartłomiej Sadowski, Dominik Mierzwa, Seongsoo Kang, Marek Grzybowski, evgen M. Poronik, Andrzej L. Sobolewski, Dongho Kim, Daniel T. Gryko
Chem. Commun. 2022, 58, 3697-3700
DOI: 10.1039/D1CC06863F
-
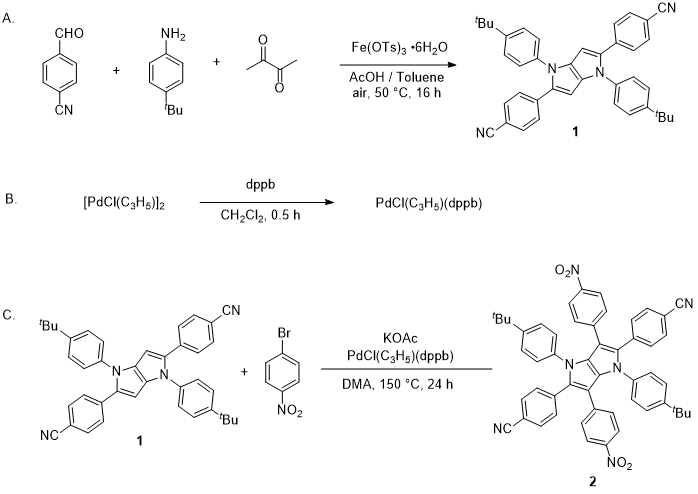
Synthesis of Tetraaryl-, Pentaaryl-, and Hexaaryl-1,4-dihydropyrrolo[3,2-b]pyrroles
Maciej Krzeszewski, Mariusz Tasior, Marek Grzybowski, Daniel T. Gryko
Org. Synth. 2021, 98, 242-262
DOI: 10.15227/orgsyn.098.0242
-
Covalent Self-Labeling of Tagged Proteins with Chemical Fluorescent Dyes in BY-2 Cells and Arabidopsis Seedlings
Ryu J Iwatate, Akira Yoshinari, Noriyoshi Yagi, Marek Grzybowski, Hiroaki Ogasawara, Mako Kamiya, Toru Komatsu, Masayasu Taki, Shigehiro Yamaguchi, Wolf B Frommer, Masayoshi Nakamura
Plant Cell 2020, 32, 3081-3094
DOI: 10.1105/tpc.20.00439
-
Method for the Large-Scale Synthesis of Multifunctional 1,4-Dihydro-pyrrolo[3,2-b]pyrroles
Mariusz Tasior, Olena Vakuliuk, Daiki Koga, Beata Koszarna, Krzysztof Górski, Marek Grzybowski, Łukasz Kielesiński, Maciej Krzeszewski, Daniel T. Gryko
J. Org. Chem. 2020, 85, 13529–13543
DOI: 10.1021/acs.joc.0c01665
-
Effects of Amino Group Substitution on the Photophysical Properties and Stability of Near-Infrared Fluorescent P-Rhodamines
Marek Grzybowski, Masayasu Taki, Keiji Kajiwara, Shigehiro Yamaguchi
Chem. Eur. J. 2020, 26, 7912-7917
DOI: 10.1002/chem.202000957
-
Synthetic Applications of Oxidative Aromatic Coupling—From Biphenols to Nanographenes
Marek Grzybowski, Bartłomiej Sadowski, Holger Butenschön, Daniel T. Gryko
Angew. Chem. Int. Ed. 2019, 59, 2998-3027
DOI: 10.1002/anie.201904934
-
A Highly Photostable Near-Infrared Labeling Agent Based on a Phospha-rhodamine for Long-Term and Deep Imaging
Marek Grzybowski, Masayasu Taki, Kieko Senda, Yoshikatsu Sato, Tetsuro Ariyoshi, Yasushi Okada, Ryosuke Kawakami, Takeshi Imamura, Shigehiro Yamaguchi
Angew. Chem. Int. Ed. 2018, 57, 10137-10141
DOI: 10.1002/anie.201804731
-
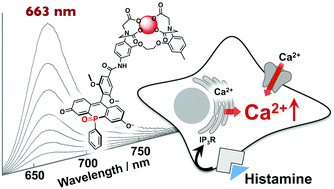
A far-red fluorescent probe based on a phospha-fluorescein scaffold for cytosolic calcium imaging
Hiroaki Ogasawara, Marek Grzybowski, Riho Hosokawa, Yoshikatsu Sato, Masayasu Taki, Shigehiro Yamaguchi
Chem. Commun. 2018, 54, 299-302
DOI: 10.1039/C7CC07344E
-
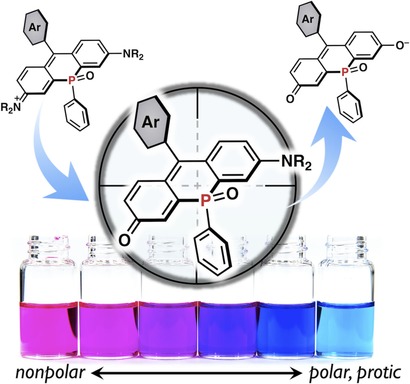
Selective Conversion of P=O-Bridged Rhodamines into P=O-Rhodols: Solvatochromic Near-Infrared Fluorophores
Marek Grzybowski, Masayasu Taki, Shigehiro Yamaguchi
Chem. Eur. J. 2017, 23, 13028-13032
DOI: 10.1002/chem.201703456
-
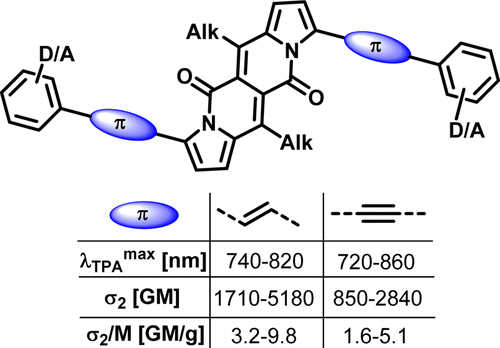
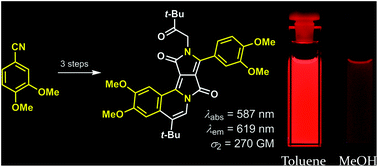
π-Expanded Dipyrrolonaphthyridinediones with Large Two-Photon Absorption Cross-Section Values
Bartłomiej Sadowski, Hanayo Kita, Marek Grzybowski, Kenji Kamada, Daniel T. Gryko
J. Org. Chem. 2017, 82, 7254–7264
DOI: 10.1021/acs.joc.7b00831
-
Synthesis and optical properties of water-soluble diketopyrrolopyrroles
Marek Grzybowski, Eliza Glodkowska-Mrowka, Guillame Clermont, Mireille Blanchard-Desce, Daniel T. Gryko
Chem. Heterocycl. Comp. 2017, 53, 72–77
DOI: 10.1007/s10593-017-2023-y
-
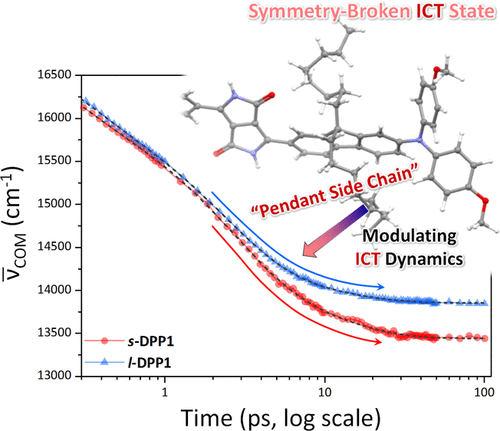
Modulation of Symmetry-Breaking Intramolecular Charge-Transfer Dynamics Assisted by Pendant Side Chains in π-Linkers in Quadrupolar Diketopyrrolopyrrole Derivatives
Woojae Kim, Jooyoung Sung, Marek Grzybowski, Daniel T. Gryko, Dongho Kim
J. Chem. Phys. Lett. 2016, 7, 3060–3066
DOI: 10.1021/acs.jpclett.6b01248
-
Z-Shaped Pyrrolo[3,2-b]pyrroles and Their Transformation into π-Expanded Indolo[3,2-b]indoles
Rafał Stężycki, Marek Grzybowski, Guillaume Clermont, Mireille Blanchard-Desce, Daniel T. Gryko
Chem. Eur. J. 2016, 22, 5198-5203
DOI: 10.1002/chem.201505052
-
Dipyrrolonaphthyridinediones – structurally unique cross-conjugated dyes
Marek Grzybowski, Irena Deperasińska, Maciej Chotkowski, Marzena Banasiewicz, Artur Makarewicz, Bolesław Kozankiewicz, Daniel T. Gryko
Chem. Commun. 2016, 52, 5108-5111
DOI: 10.1039/C6CC01017B
-
Solvatofluorochromic, non-centrosymmetric π-expanded diketopyrrolopyrrole
Marek Grzybowski, Artur Jeżewski, Irena Deperasińska, Daniel H. Friese, Marzena Banasiewicz, Vincent Hugues, Bolesław Kozankiewicz, Mireille Blanchard-Desce, Daniel T. Gryko
Org. Biomol. Chem. 2016, 14, 2025-2033
DOI: 10.1039/C5OB02583D
-
Phospha-fluorescein: a red-emissive fluorescein analogue with high photobleaching resistance
Aiko Fukazawa, Shinji Suda, Masayasu Taki, Eriko Yamaguchi, Marek Grzybowski, Yoshikatsu Sato, Tetsuya Higashiyama, Shigehiro Yamaguchi
Chem. Commun. 2016, 52, 1120-1123
DOI: 10.1039/C5CC09345G
-
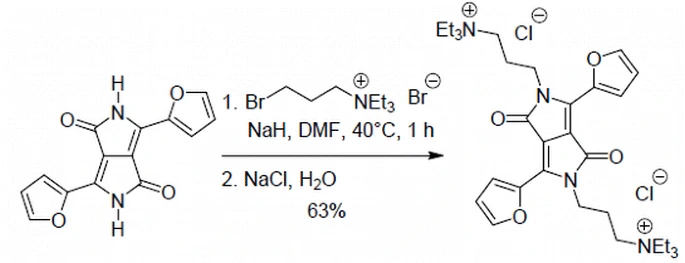
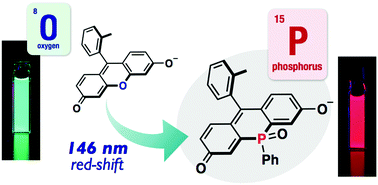
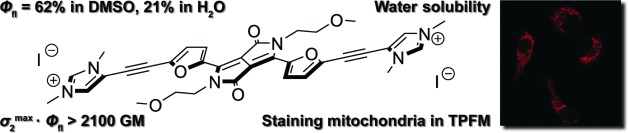
Polar Diketopyrrolopyrrole-Imidazolium Salts as Selective Probes for Staining Mitochondria in Two-Photon Fluorescence Microscopy
Marek Grzybowski, Eliza Glodkowska-Mrowka, Vincent Hugues, Wojciech Brutkowski, Mireille Blanchard-Desce, Daniel T. Gryko
Chem. Eur. J. 2015, 21, 9101-9110
DOI: 10.1002/chem.201500738
-
Diketopyrrolopyrroles: Synthesis, Reactivity, and Optical Properties
Marek Grzybowski, Daniel T. Gryko
Adv. Opt. Mater. 2015, 3, 280-320
DOI: 10.1002/adom.201400559
-
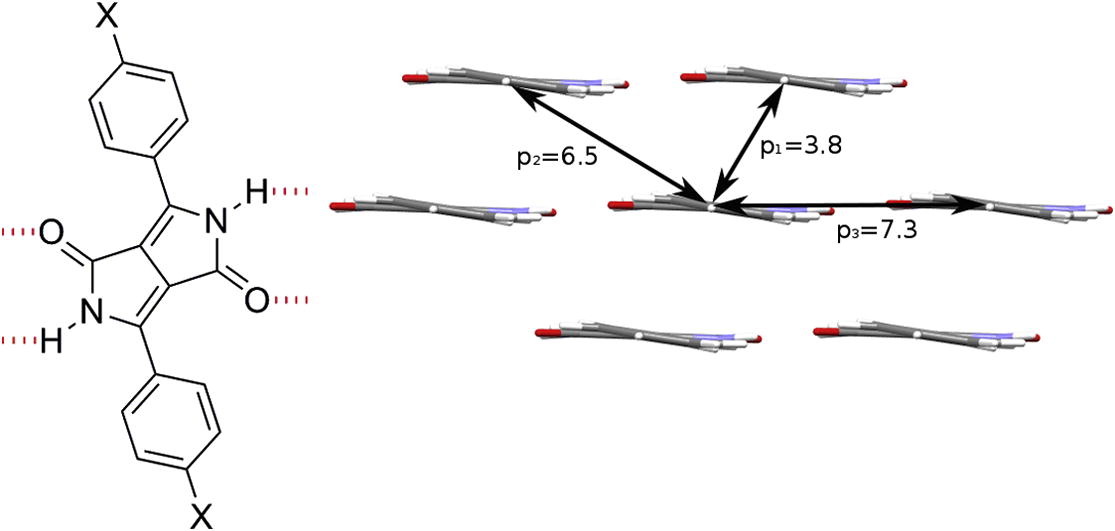
Hydrogen-bonded diketopyrrolopyrrole (DPP) pigments as organic semiconductors
Eric Daniel Głowacki, Halime Coskun, Martin A. Blood-Forsythe, Uwe Monkowius, Lucia Leonat, Marek Grzybowski, Daniel Gryko, Matthew Schuette White, Alán Aspuru-Guzik, Niyazi Serdar Sariciftci
Organic Electronics 2014, 15, 3521-3528
DOI: 10.1016/j.orgel.2014.09.038
-
Two-Photon-Induced Fluorescence in New π-Expanded Diketopyrrolopyrroles
Marek Grzybowski, Vincent Hugues, Mireille Blanchard-Desce, Daniel T. Gryko
Chem. Eur. J. 2014, 20, 12493-12501
DOI: 10.1002/chem.201402569
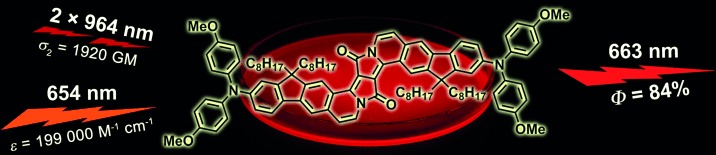
-
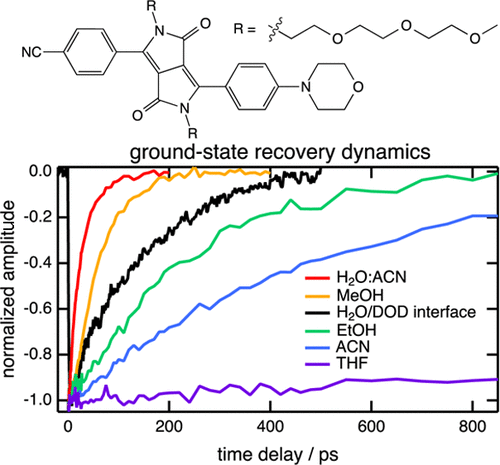
Excited-State Dynamics of an Environment-Sensitive Push–Pull Diketopyrrolopyrrole: Major Differences between the Bulk Solution Phase and the Dodecane/Water Interface
Sabine Richert, Sandra Mosquera Vazquez, Marek Grzybowski, Daniel T. Gryko, Alexander Kyrychenko, Eric Vauthey
J. Phys. Chem. B 2014, 118, 9952–9963
DOI: 10.1021/jp506062j
-
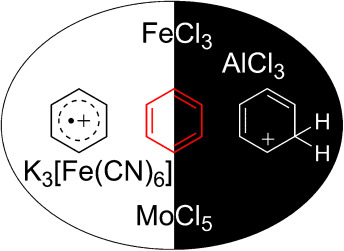
Comparison of Oxidative Aromatic Coupling and the Scholl Reaction
Marek Grzybowski, Kamil Skonieczny, Holger Butenschön, Daniel T. Gryko
Angew. Chem. Int. Ed. 2013, 52, 9900-9930
DOI: 10.1002/anie.201210238
-
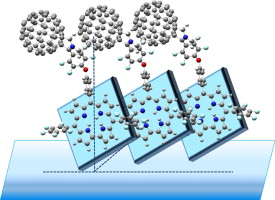
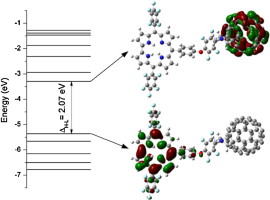
Spectral studies of molecular orientation in corrole-fullerene thin films
B. Bursa, D. Wróbel, K. Lewandowska, A. Graja, M. Grzybowski, D.T. Gryko
Synthetic Metals 2013, 176, 70-76
DOI: 10.1016/j.synthmet.2013.05.015
-
Absorption and emission properties of the corrole–fullerene dyad
Kornelia Lewandowska, Bolesław Barszcz, Andrzej Graja, Bartosz Bursa, Andrzej Biadasz, Danuta Wróbel, Waldemar Bednarski, Stefan Waplak, Marek Grzybowski, Daniel T. Gryko
Synthetic Metals 2013, 166, 70-76
DOI: 10.1016/j.synthmet.2013.01.014
-
Vibrational properties of new corrole–fullerene dyad and its components
Kornelia Lewandowska, Bolesław Barszcz, Jacek Wolak, Andrzej Graja, Marek Grzybowski, Daniel T. Gryko
Dyes Pigm. 2013, 96, 249-255
DOI: 10.1016/j.dyepig.2012.07.024
-
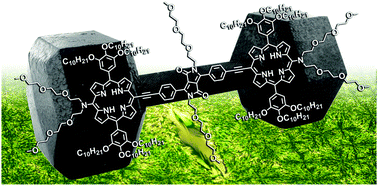
Strong two-photon absorption enhancement in a unique bis-porphyrin bearing a diketopyrrolopyrrole unit
Agnieszka Nowak-Król, Marek Grzybowski, Jerzy Romiszewski, Mikhail Drobizhev, Geoffrey Wicks, Maciej Chotkowski, Aleksander Rebane, Ewa Górecka, Daniel T Gryko
Chem. Commun. 2013, 49, 8368-8370
DOI: 10.1039/C3CC44728F
-
Bright, Color-Tunable Fluorescent Dyes Based on π-Expanded Diketopyrrolopyrroles
Marek Grzybowski, Eliza Glodkowska-Mrowka, Tomasz Stoklosa, Daniel T. Gryko
Org. Lett. 2012, 14, 2670–2673
DOI: 10.1021/ol300674v